Formation of kinetically trapped small clusters of PEGylated gold nanoparticles revealed by the comb
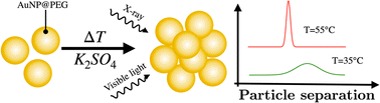 |
26/10/2022. Gold nanoparticles coated with polyethylene glycol (PEG) are able to form clusters due to the collapse of the surface-grafted polymer chains when the temperature and ion concentration of the aqueous medium are increased. The chain collapse reduces the steric repulsion, leading to particle aggregation. In this work, we combine small angle X-ray scattering (SAXS) and visible light spectroscopy to elucidate the structure of the developing clusters. The structure derived from the SAXS measurements reveals a decrease in interparticle distance and drastic narrowing of its distribution in the cluster, indicating restricted particle mobility and displacement within the cluster. Surprisingly, instead of forming a large crystalline phase, the evolving clusters are composed of about a dozen particles. The experimental optical extinction spectra measured during cluster formation can be very well reproduced by optical simulations based on the SAXS-derived structural data.
Thanks to Daniel and Andras from the Centre for Energy Research (Budapest, Hungary) for this nice collaboration. |